The correct answer is "the volume increases".
In fact, Charle's law states that when the pressure of a gas stays constant, the ratio between the temperature and the volume of the gas is constant:
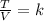
where T is the gas temperature and V is its volume. We see from the formula that, if the temperature T increases, since the ratio must remain constant, the volume of the gas V increases as well.