Answer: Option (a) is the correct answer.
Step-by-step explanation:
The given overall reaction will be as follows.
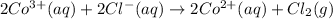
It is given that standard potential of the cell is 0.46 V.
Hence, the oxidation and reduction half reactions of the cell are as follows.
Reduction-half reaction:
 ,
,
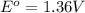
Oxidation-half reaction:
 ,
,
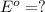
Now, expression of
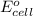 of this reaction is as follows.
of this reaction is as follows.
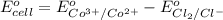
Putting the given values into the above formula as follows.
0.46 V =
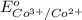 - 1.36 V
- 1.36 V
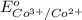 = 1.82 V
= 1.82 V
Thus, we can conclude that standard potential of the given half cell is 1.82 V.