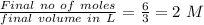Answer:
2 M NaOH solution
Step-by-step explanation:
Initial concentration of NaOH = 12 M
Initial volume of solution = 0.5
Initial number of moles = Molarity×Volume = 12×0.5 = 6 moles
Note: On dilution the number of moles does not change.
Initial number of moles = final number of moles.
Final number of moles = 6 moles
Final volume of solution = 3 L
Final concentration (in Molarity) =