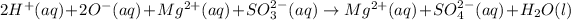Answer: This a redoc reaction.
The total ionic equation in separated aqueous solution will be,
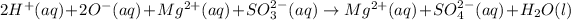
Explanation :
The given reaction is a redox reaction in which the oxygen shows reduction and sulfur shows oxidation reaction.
In the net ionic equations, we are not include the spectator ions in the equations.
Spectator ions : The ions present on reactant and product side which do not participate in a reactions. The same ions present on both the sides.
The given balanced ionic equation will be,
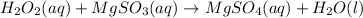
The total ionic equation in separated aqueous solution will be,