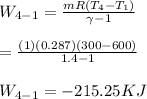Answer:
Step-by-step explanation:
Check attachment for p-v Diagram
a.)
Calculate the maximum temperature of the cycle by using ideal gas equation:
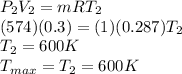
Calculate the minimum temperature of the cycle:
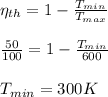
b.)
Calculate the volume at the beginning of the isothermal expansion by using the following expression:
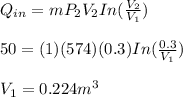
Calculate the pressure at the beginning of the isothermal expansion by using the following expression:

c.)
Process 1-2:
Heat addition for the Process 1-2 is
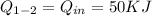
Calculate the work transfer for the process 1-2:
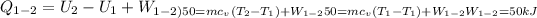
Process 2-3:It is an Adiabatic Process:
Heat transfer for the Process 2-3 is
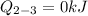
Calculate the work transfer for the process 2-3:
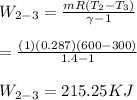
Process 3-4:
Calculate the heat transfer for the process 3-4:
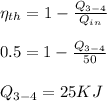
Calculate the work transfer for the process 3-4:
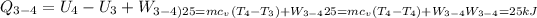
Process 4-1: It is an Adiabatic Process:
Heat transfer for the process 4-1 is
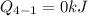
Calculate the work transfer for the process 4-1: