Answer:
The coefficient of
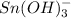 is 3 in the balanced redox reaction.
is 3 in the balanced redox reaction.
Step-by-step explanation:
Oxidation reaction is defined as the chemical reaction in which an atom loses its electrons. The oxidation number of the atom gets increased during this reaction.
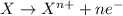
Reduction reaction is defined as the chemical reaction in which an atom gains electrons. The oxidation number of the atom gets reduced during this reaction.
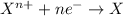
For the given chemical reaction:
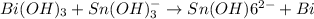
The half cell reactions for the above reaction follows:
Oxidation half reaction:
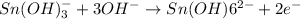
Reduction half reaction:

To balance the oxidation half reaction must be multiplied by 3 and reduction half reaction must be multiplied by 2 thus, the balanced equation is:-
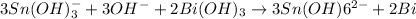
The coefficient of
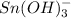 is 3 in the balanced redox reaction.
is 3 in the balanced redox reaction.