Answer:
T_finalmix = 59.5 [°C].
Step-by-step explanation:
In order to solve this problem, a thermal balance must be performed, where the heat is transferred from water to methanol, at the end the temperature of the water and methanol must be equal once the thermal balance is achieved.
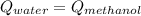
where:
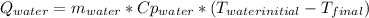
mwater = mass of the water = 0.4 [kg]
Cp_water = specific heat of the water = 4180 [J/kg*°C]
T_waterinitial = initial temperature of the water = 85 [°C]
T_finalmix = final temperature of the mix [°C]
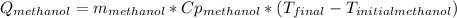
Now replacing:
![0.4*4180*(85-T_(final))=0.4*2450*(T_(final)-16)\\142120-1672*T_(final)=980*T_(final)-15680\\157800=2652*T_(final)\\T_(final)=59.5[C]](https://img.qammunity.org/2022/formulas/physics/high-school/tn2d8p5afhba61jqr51spgo7h0w6t75m28.png)