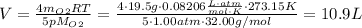Answer:
10.9 L
Step-by-step explanation:
Let's begin by introducing the strategy to solve this problem:
- Write a chemical reaction and make sure it's balanced;
- Identify STP (standard temperature and pressure, that is, T = 273.15 K and p = 1.00 atm);
- Find moles of oxygen;
- From the stoichiometry of the equation, identify the number of moles of ammonia;
- Convert moles of ammonia into volume using the ideal gas law pV = nRT.
(a) The first step is already fulfilled: the reaction is balanced and it shows that 4 moles of ammonia react with 5 moles of oxygen.
(b) Given the following variables:
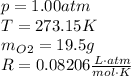
(c) Find moles of oxygen dividing mass of oxygen by its molar mass:
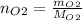
Here molar mass of oxygen is:
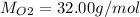
(d) From stoichiometry, dividing moles of ammonia by its stoichiometric coefficient would be equal to the ratio of moles of oxygen divided by its stoichiometric coefficient:
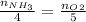
Rearrange the equation to obtain moles of ammonia:
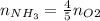
(e) Solve pV = nRT for volume of ammonia:
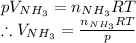
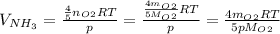
Substitute the given data to obtain the final answer!