Answer: The balanced molecular equation is written below.
Step-by-step explanation:
A molecular equation is defined as the chemical equation in which the ionic compounds are written as molecules rather than component ions.
When zinc (II) nitrate reacts with sodium hydroxide, it leads to the formation of white precipitate of zinc (II) hydroxide and an aqueous solution of sodium nitrate.
The balanced chemical equation for the above reaction follows:
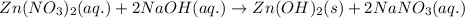
By Stoichiometry of the reaction:
1 mole of aqueous solution of zinc (II) nitrate reacts with 1 mole of aqueous solution of sodium hydroxide to produce 1 mole of solid zinc hydroxide and 2 moles of aqueous solution of sodium nitrate
Hence, the balanced molecular equation is written above.