Answer:
1.34352 kg
Step-by-step explanation:
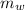 = Mass of water falling = 1 kg
= Mass of water falling = 1 kg
h = Height of fall = 0.1 km
 = Change in temperature = 0.1
= Change in temperature = 0.1
c = Specific heat of water = 4186 J/kg K
g = Acceleration due to gravity = 9.81 m/s²
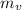 = Mass of water in the vessel
= Mass of water in the vessel
Here the potential energy will balance the internal energy
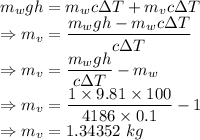
Mass of the water in the vessel is 1.34352 kg