The given question is incomplete. But the complete question is this:
A mixture of
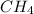 and
and
 is passed over a nickel catalyst at 1000 K. The emerging gas is collected in a 5.00-L flask and is found to contain 8.62 g of CO, 2.60 g of
is passed over a nickel catalyst at 1000 K. The emerging gas is collected in a 5.00-L flask and is found to contain 8.62 g of CO, 2.60 g of
 , 43.0 g of
, 43.0 g of
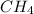 , and 48.4 g of
, and 48.4 g of
 . Assuming that equilibrium has been reached, calculate
. Assuming that equilibrium has been reached, calculate
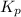 for the reaction.
for the reaction.
Step-by-step explanation:
As the given reaction is as follows.
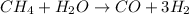
And, we know that
No. of moles =

Therefore, calculate the moles as follows.
Moles of
 =
=
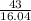
= 2.6808 mol
Moles of
 =
=
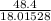
= 2.6866 mol
Moles of CO =
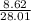
= 0.307747 mol
Moles of
 =
=
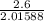
= 1.2897 mol
As, we know that
Concentration =
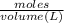
Given volume = 5 L
Hence, calculate the concentration of given species as follows.
Conc. of
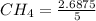
= 0.5361
Conc. of
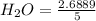
= 0.5373
Conc. of CO =
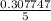
= 0.06155
and, Conc. of
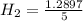
= 0.2579
Now, expression for equilibrium constant for the given reaction is as follows.
![K_(c) = ([CO][H_2]^(3))/([CH_4][H_2O] )](https://img.qammunity.org/2020/formulas/chemistry/high-school/ssz6fm9wo8kl437fgbtwz6cct1mcl9vhb0.png)
Now, putting the given values into the above formula as follows.
![K_(c) = ([0.06155][0.2579]^(3))/([0.5361][0.5373])](https://img.qammunity.org/2020/formulas/chemistry/high-school/3mynbku6gu2iazdqpf5og9hnpse5ce8rxt.png)
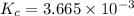
Also, we know that
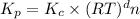
Consider the equation
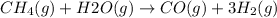
Calculate change in moles of gas as follows.
change in gas moles (dn) = 1 + 3 - 1 - 1
dn = 2
As,
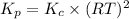
It is given that,
T = 1000 K , R = 0.0821
So,
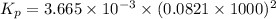
 = 24.70
= 24.70
Thus, we can conclude that value of
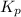 for the reaction is 24.70.
for the reaction is 24.70.