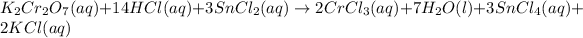Answer:
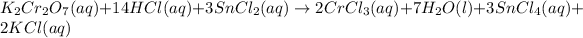
Step-by-step explanation:
The products of this reaction are given by:
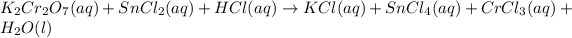
Firstly, dichromate anion becomes chromium(III) cation, let's write this change:
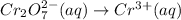
The following steps should be taken:
- balance the main element, chromium: multiply the right side by 2 to get 2 chromium species on both side:
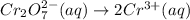
- balance oxygen atoms by adding 7 water molecules on the right:
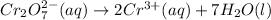
- balance the hydrogen atoms by adding 14 protons on the left:
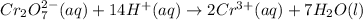
- balance the charge (the total net charge on the left is 12+, on the right we have 6+, so 6 electrons are needed on the left):
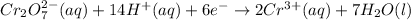
Similarly, tin(II) cation becomes tin(IV) cation:
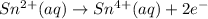
Now that we have the two half-equations, multiply the second one by 3, so that it also has 6 electrons that will be cancelled out upon addition of the two half-equations:
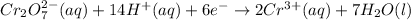
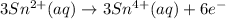
Add them together:
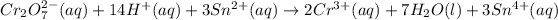
Adding the ions spectators: