Answer:
i=1.62 .
Step-by-step explanation:
Let, i be the Van't Hoff Factor.
Moles of benzamide,=
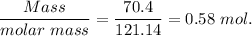
Molality of solution, m=
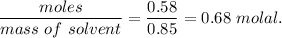
Now, we know
Depression in freezing point,
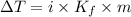 .....1
.....1
It is given that,
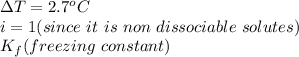
Putting all these values we get,
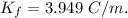
Now, moles of ammonium chloride=
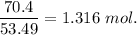
molality =
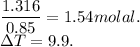
Putting all these values in eqn 1.
We get,
i=1.62 .
Hence, this is the required solution.