Answer:
correct option is c. 31.0°C
Step-by-step explanation:
given data
copper of mass = 100 g
electric drill = 1/2"
power = 40.0 W
time = 30 s
C copper = 387 J/kgC
to find out
copper's increase in temperature
solution
we get here energy that is express a s
energy = 40 W × 30 s
energy = 1200 Watt seconds
and heat acquired by drill is here as
heat acquired = 100 × T × 387
here temperature rise in copper mass as
temperature rise in copper mass =
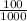 × T × 387
× T × 387
temperature rise in copper mass = 38.7 × T Watt second
we know that all the energy from the drill heats the copper
so we can say
38.7 × T = 1200
T = 31°C
so correct option is c. 31.0°C