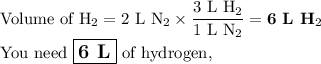Answer:
Step-by-step explanation:
We can use Gay-Lussac's Law of Combining Volumes to solve this problem.
Gases at the same temperature and pressure react in the same ratios as their coefficients in the balanced equation.
1. Write the chemical equation.
Ratio: 1 L 3 L
N₂ + 3H₂ ⟶ 2NH₃
V/L: 2
2. Calculate the volume of H₂.
According to Gay-Lussac, 3 L of H₂ react with 1 L of N₂.
Then, the conversion factor is (3 L H₂/1 L N₂).