Answer:
373.1 mL of AgCN (aq) must be poured into your electrolysis vat to ensure you have sufficient Ag to plate all of the forks.
Step-by-step explanation:
Mass of silver to be precipitated on ecah spoon = 0.500 g
Number of silver spoons = 250
Total mass of silver = 250 × 0.500 g = 125 g
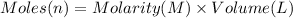
Moles of AgCN = n =
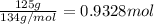
Volume of AgCN solution =V
Molarity of the AgCN = 2.50 M
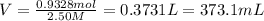
(1 L = 1000 mL)
373.1 mL of AgCN (aq) must be poured into your electrolysis vat to ensure you have sufficient Ag to plate all of the forks.