Answer:
Yes
Step-by-step explanation:
Before dilution:
Volume of NaCl = 10 mL
Concentration of NaCl = 0.5 M
Number of moles = Molarity*Volume = 0.5*10 = 5 millimoles.
Note that number of moles of NaCl does not change on dilution as we are only adding water.
After dilution:
Volume of NaCl = 100 mL
Number of moles = 5 millimoles (no change)
New Concentration = Number of moles per volume in litres =
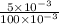 = 0.05 Molar
= 0.05 Molar
Hence the concentration became one-tenth of the initial concentration after dilution.