Answer:
The balanced chemical equation is given as:
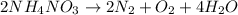
Step-by-step explanation:
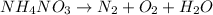
During the balancing of the chemical reaction, balance all the atoms of every atoms except oxygen element.
There are 2 nitrogen atoms on both sides. Nitrogen atom is balanced.
There are 4 hydrogen atoms on left side and 2 hydrogen atom on right side.Write 2 in front of
 to balance the hydrogen atoms.
to balance the hydrogen atoms.
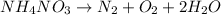
Now ,see the number of oxygen atoms on both sides. Here on both sides oxygen atoms oxygen atom are same.
So, write 2 in front of
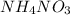 , 2 in front of
, 2 in front of
 and 4 in front of
and 4 in front of
 .
.
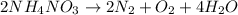
All the atoms of all each and every element is balanced in the chemical equation.