Answer:
Number of atoms=
 atoms
atoms
Step-by-step explanation:
1 mole is the mass of substance which contain

particles of that substance.Its symbol is 'n'.
Here the quantity
 is called Avogadro number and it is denoted by
is called Avogadro number and it is denoted by
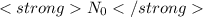 .
.
Mass of the substance in grams is called is molar mass.It is denoted by'M'
Moles can be calculated by :
1)
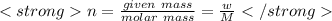
2)
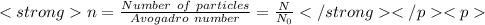
compare 1) and 2) formula, we get
![<strong>\frac{N}{N_(0)=(w)/(M)</strong>[tex].................(i)</p><p>For<strong> silver (Ag), M=107.86 g</strong></p><p><strong>w=18 g </strong>(given) and [tex]<strong>{N_(0)=6.022* 10^(23)</strong>]()
Put the values of w,M and
![{N_(0)[tex] in equation (i) and solve for N</p><p>[tex]\<strong>frac{N}{6.022* 10^(23)}=(18)/(107.86)</strong>]()
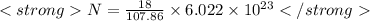
![<strong>N= 1.004* 10^(23)</strong>[tex]</p><p><strong>Hence Number of atoms of silver</strong>= [tex]<strong>1.004* 10^(23)</strong>](https://img.qammunity.org/2020/formulas/chemistry/middle-school/bzfkltigjkc6kqvg9hxn4rx6z5d6x69zbv.png) atoms
atoms