Answer:
The molar mass of
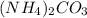 is 96.8 g/mol
is 96.8 g/mol
Step-by-step explanation:
The given molecular formula -
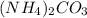
Individual molar masses of each element in the compound is as follows.
Molar mass of nitrogen - 14.01 g/mol
Molar mass of of hydrogen = 1.008g/mol
Molar mass of carbon = 12.01 g/mol
Molar mass of oxygen =16.00 g/mol
Molar mass of
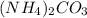 is
is
![2*[1(14.01)+4(1.008)]+1(12.01)+3(16.00)= 96.8g/mol](https://img.qammunity.org/2020/formulas/chemistry/middle-school/nswi6x72mvv25gtcqdkc1yk8dk3f78w29c.png)
Therefore,The molar mass of
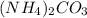 is 96.8 g/mol
is 96.8 g/mol