Answer:
The number of atoms present in one unit of the following compounds is:
a). Potassium Iodide , KI = 2
b).Sodium Sulfide,
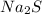 = 3
= 3
c). Silicon Dioxide ,
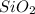 = 3
= 3
d). Carbonic Acid ,
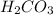 = 6
= 6
Step-by-step explanation:
Atomicity : It is defined as the number of atoms that are present in a given molecule/compound.
Atom : The smallest unit of matter is called atom. For e.g O is atom of oxygen but
 is not an atom , it is molecule of oxygen .
is not an atom , it is molecule of oxygen .
 molecule has 2 atoms of Oxygen
molecule has 2 atoms of Oxygen
Similarly Na , K , Fe are atoms but
 ,
,
 ,
,
 ,
,
 are molecules
are molecules
a).Potassium Iodide
KI = 1 atom of K + 1 atom of I
Total atoms = 2
b) Sodium Sulfide
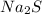 = 2 atoms of Na + 1 atom of S
= 2 atoms of Na + 1 atom of S
Total atoms = 3
c) Silicon Dioxide
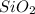 = 1 atom of Si + 2 atoms of O
= 1 atom of Si + 2 atoms of O
Total atoms = 3
d) Carbonic Acid
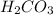 = 2 atom of H + 1 atom of C + 3 atom of O
= 2 atom of H + 1 atom of C + 3 atom of O
= 2+1+3
Total atoms = 6