Answer:
The pressure of the gas increases by the four times.
Step-by-step explanation:
According to the Gay-Lussac's law
The relationship between pressure and temperature is as follows.
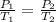 \
\
From the given data, we considered 1 is a initial temperature and 4 is final temperature. Initial pressure is considered as
 .
.
Let's find the final pressure of the gas.
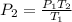
Substitute the temperature value.
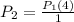

Therefore, The pressure of the gas increases by the four times.