Answer:
The temperature change of the copper is greater than the temperature change of the water.
Step-by-step explanation:
deltaQ = mc(deltaT)
Where,
delta T = change in the temperature
m =mass
c = heat capacity
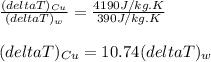
The temperature change in the copper is nearly 11 times the temperature change in the water.
So, the correct option is,
The temperature change of the copper is greater than the temperature change of the water.
Hope this helps!