Answer:
Assumption:
1. The kinetic and potential energy changes are negligible
2. The cylinder is well insulated and thus heat transfer is negligible.
3. The thermal energy stored in the cylinder itself is negligible.
4. The process is stated to be reversible
Analysis:
a. This is reversible adiabatic(i.e isentropic) process and thus
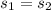
From the refrigerant table A11-A13
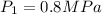
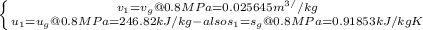
sat vapor
m=
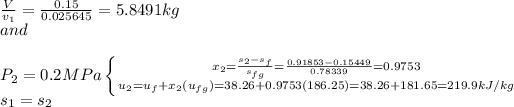
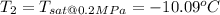
b.) We take the content of the cylinder as the sysytem.
This is a closed system since no mass leaves or enters.
Hence, the energy balance for adiabatic closed system can be expressed as:
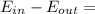 ΔE
ΔE
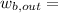 ΔU
ΔU
![w_(b, out) =m([tex]u_(1) -u_{2](https://img.qammunity.org/2020/formulas/engineering/college/1z0xj4kzwqyjcaugy2w2mt0usvjk0g79zp.png) )
)
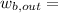 workdone during the isentropic process
workdone during the isentropic process
=5.8491(246.82-219.9)
=5.8491(26.91)
=157.3993
=157.4kJ