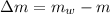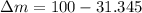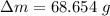Answer:
Step-by-step explanation:
Given:
- mass of ice,
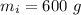
- initial temperature of ice,
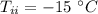
- mass of water added,
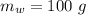
- initial temperature of water,
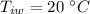
- specific heat capacity of ice,
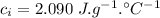
- specific heat capacity of water,
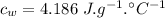
- latent heat of fusion,
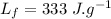
According to question a part of water freezes while the other remains liquid:
So,
final temperature of the mixture,
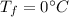
Now using the equation of heat:
Heat required by the total ice of given temperature to melt completely:
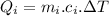
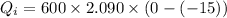
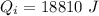 .....................................(1)
.....................................(1)
Heat released by the total mass of given water to come to freezing point:
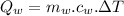
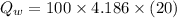
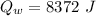 ...............................................(2)
...............................................(2)
Now from the eq. (1) & (2) the difference in heat is equivalent to the mass of water frozen.
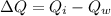
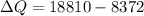
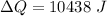
Now the mass of water frozen:
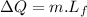
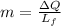
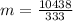
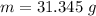 of more ice is formed from the water.
of more ice is formed from the water.
Mass of remaining liquid: