Answer:
Step-by-step explanation:
Given that the tank is insulated and rigid, heat transfer to the surrounding is zero and there is no change in volume of system(container).
p1=400kpa, T1= 40°c= 313k, p2=800kpa
volume of container = volume of cube= a³=1³=1m³
Air is acting as an ideal gas, hence, applying ideal gas equatin
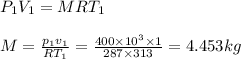 R= 287J/kg.k for air
R= 287J/kg.k for air
a.) Process 1-2 constant volume process v1=v2
P∝T=
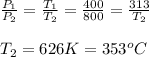
b.) According to first law of thermodynamics
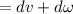
δQ=0(insulated container)
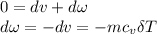
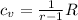
where r= specific heat ratio; for air, r=1.4, R= 0.287KJ/kg.k
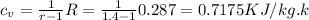
Then
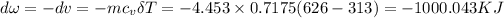
The negative sign means work done of system is in reverse.
c.) Entropy generation = change in system + change in surrounding
change in surrounding is zero
change in system=
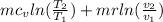
v1=v2; v2/v1=1; ln(1)=0
entropy gen= 4.453*0.7175*ln(626/313) + 0 =2.215KJ/k