Answer:
The type of reaction for the following equation is combustion equation.
Step-by-step explanation:
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
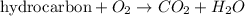
The reaction given to us:
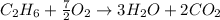
When 1 mole of ethane reacts with 7/2 moles of oxygen gas it gives 3 moles of water and 2 moles of carbon dioxide gas.
The type of reaction for the following equation is combustion equation.