Answer:
520 kJ·mol⁻¹
Step-by-step explanation:
You calculate the energy required to break all the bonds in the reactants. Then you subtract the energy to break all the bonds in the products.
SF₄(g) + 2H₂O(g) ⟶ SO₂(g) + 4HF(g); ΔH = –123 kJ
Bonds: 4S-F + 4O-H 2S=O 4H-F
D/kJ·mol⁻¹: 327 467 x 565
The formula relating ΔᵣH and bond dissociation energies (D) is
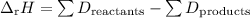
(Note: This is an exception to the rule. All other thermochemical reactions are “products – reactants”. With bond energies, it’s “reactants – products”. The reason comes from the way we define bond energies.)
Σ(Dreactants) = 4 × 327 + 4 × 467 = 3176 kJ
Σ(Dproducts) = 2x +4 × 565 = (2x + 2260) kJ
-123 =3176 - (2x + 2260)
-123 = 3176 - 2x - 2260
-123 = 916 - 2x
2x = 1039
x = 520 kJ·mol⁻¹
The average bond energy of the S=O bonds in SO₂ is 520 kJ/mol.