The bond shared between Oxygen and Nitrogen is the covalent bond.
Option C
Step-by-step explanation:
The bond formed between oxygen and nitrogen atom is due to the sharing of electrons between the two atoms. The electronegativity difference between the nitrogen and the oxygen atom is not enough to cause an ionic interaction between them. Hence, they are incapable to form an ionic bond.
For eg-
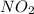
In this compound, nitrogen atom forms a double bond (which is a covalent double bond) with one atom of oxygen and a coordinate bond with the other atom of oxygen.