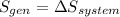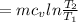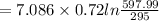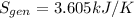Answer:
m = 7.086 kg
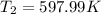
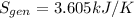
Step-by-step explanation:
Given data:
volume of sir - 3 m^3
temperature is t = 295 K
Pressure is = 200 kPa
for air , R = 0.287 kJ/kg k
a) from ideal gas equation we have
PV = mRT
solving for m
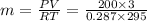
m = 7.086 kg
b) by energy balance principle
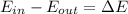
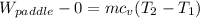
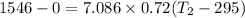
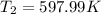
C)ENTROPY