Answer : The heat value of 'q' in exothermic reaction is -8900 kJ
Explanation :
First we have to calculate the number of moles of methane.
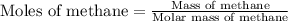
Molar mass of methane = 16 g/mole
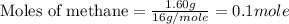
Now we have to calculate the heat released in the reaction.
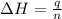
or,
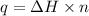
where,
 = enthalpy change = 890.0 kJ/mol
= enthalpy change = 890.0 kJ/mol
q = heat released = ?
n = number of moles of methane = 0.1 mol
Now put all the given values in the above formula, we get:
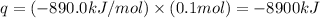
Therefore, the heat value of 'q' in exothermic reaction is -8900 kJ