Answer:
-18.042
Step-by-step explanation:
A photon is refer to as a particle of light with a discrete bundle of electromagnetic energy. Photon is always in motion and in a vacuum with a constant light speed to all viewers. The amount of energy of the photon (E) is calculated using:
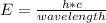
Where:
h is Planck's constant =
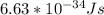
c is the speed of light = 299792458 m/s
wavelength = 219 nm =
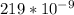 m
m
Therefore, the amount of energy of the photon is:
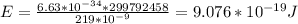
Taking the log (base 10) of the value of the energy, we have:
log (base 10)
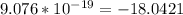
Thus, the answer in three decimal places is -18.042