Answer:
67.5°C will be the final temperature of the water.
Step-by-step explanation:
Density of water = 1 g/ml
mass = Density × Volume
Mass of 20 mL water =
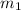
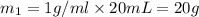
Mass of 60 mL water =
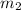
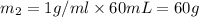
Heat gained by water at 30°C will be equal to heat lost by the water at 80°C
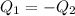
Mass of water at 30°C=
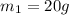
Specific heat capacity of water =
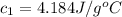
Initial temperature water at 30°C =
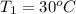
Final temperature after mixing =
 =T
=T
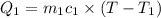
Mass of water at 80°C=
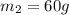
Specific heat capacity of water at 80°C=
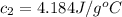
Initial temperature of the water at 80°C=
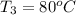
Final temperature of water after mixing=
 =T
=T
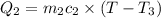
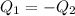
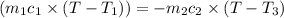
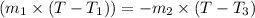
On substituting all values:
![(20 g* (T-30^oC))=-[60 g* (T-80^oC)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/htx6brtn9vpxzgl1m1k0wlb4ihzpv423e3.png)
we get, T = 67.5 °C
67.5°C will be the final temperature of the water.