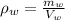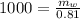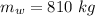Answer:
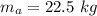 is the mass of air.
is the mass of air.
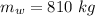 is the mass of water.
is the mass of water.
Step-by-step explanation:
Given:
- Volume of air,
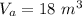
- temperature of air,
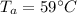
- volume of water in tub,
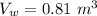
- temperature of water in the tub,
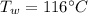
- density of air,
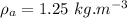
- density of water,
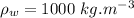
We know:
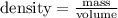
So, mass of air in the room :
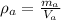
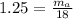
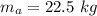
Mass of water in the tub: