Answer:
61 hours
Step-by-step explanation:
In this question we will use radioactive decay model
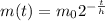
m0= 250 mg
h is the hlf life of element
m(t) is the mass left after t time
so,
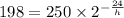
solving this equation for h
reaaranging and taking ln on both the sides we get
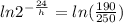
solving further we get
h= 60.62 hours ≅61 hour
the half life of element is 61 hours