Answer:
Balanced equation:
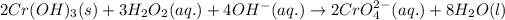
The coefficient of water is 8
Step-by-step explanation:
Oxidation:
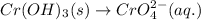
Balance O and H in basic medium:
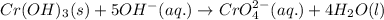
Balance charge:
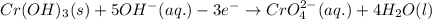 ......(1)
......(1)
Reduction:
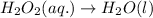
Balance O and H in basic medium:
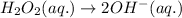
Balance charge:
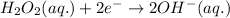 .....(2)
.....(2)
![[2* equation(1)]+[3* equation(2)]:](https://img.qammunity.org/2020/formulas/chemistry/college/tov6sj20eczfu2rth7ig2nq7cd8ws0ynjg.png)
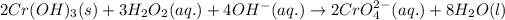
The coefficient of water is 8.