Answer:
Q =843780 J
Step-by-step explanation:
Given that,
The specific heat of a certain type of cooking oil is,
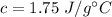
Mass of the oil, m = 2.87 kg = 2870 g
Initial temperature,
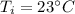
Final temperature,
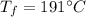
To find,
Heat energy needed to raise the temperature.
Solution,
The heat energy needed to raise the temperature in terms of specific heat is given by :

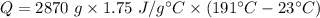
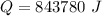
So, the heat energy needed to raise the temperature is 843780 J.