Answer : The correct option is, (2) Both electrons of Mg should be transferred to one O because the formula is MgO.
Explanation :
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
Covalent compound : It is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound.
The covalent compound are usually formed when two non-metals react.
As we know that the magnesium is an alkali metal and has 2 valence electron and oxygen is a non-metal and has 6 valence electrons.
As we know that magnesium atom can donates 2 electrons to a oxygen atom to complete an octet then it forms an ionic compound that is magnesium oxide (MgO).
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot'.
The given molecule is,
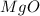
Therefore, the total number of valence electrons in
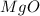 = 2 + 6 = 8
= 2 + 6 = 8
According to Lewis-dot structure, there are 2 number of bonding electrons and 6 number of non-bonding electrons.
Hence, the correct option is, (2) Both electrons of Mg should be transferred to one O because the formula is MgO.