Answer:
4.867 L of ammonia
Step-by-step explanation:
Using Haber's process to form ammonia using Nitrogen and hydrogen, the equation is :
N₂ + 3H₂ → 2NH₃
Here, 3 moles of hydrogen gas gives 2 moles of ammonia.
1 mole of any substance occupies 22.4L at STP
So, 3 x 22.4L of hydrogen gives 2 x 22.4 L of ammonia
Then 7.3 L of hydrogen will give:
=
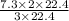
=
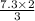
= 4.867 L of ammonia