Answer:
They move faster.
Step-by-step explanation:
Considering air as our system
When energy is transferred to air, the internal energy of the system increases and this affects the motion of the particles constituting air.
according to the equation.
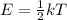
k = boltzman's constant
T= absolute temperature in kelvin
Also, Kinetic energy
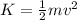
their velocity increases..
When the internal energy of the air particles increases, their temperature also increases.
This results in greater number of collisions between them and the sides of the container they are put in.
Hence, the pressure also increases, all these points indicate that particles of air move faster.