Answer:
The molar heat of combustion of hydrazine is -663.82 kJ/mole.
Step-by-step explanation:
First we have to calculate the heat gained by the calorimeter.
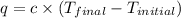
where,
q = heat gained = ?
c = specific heat =
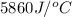
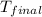 = final temperature =
= final temperature =
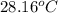
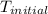 = initial temperature =
= initial temperature =
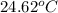
Now put all the given values in the above formula, we get:
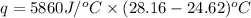
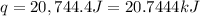
Now we have to calculate the enthalpy change during the reaction.
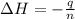
where,
 = enthalpy change = ?
= enthalpy change = ?
q = heat gained = 20.7444 kJ
n = number of moles fructose =
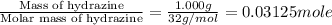
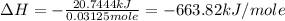
The molar heat of combustion of hydrazine is -663.82 kJ/mole.