Answer:
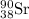
Mass defect of the oxygen nucleus is:- 0.1370 amu
Step-by-step explanation:
In a nuclear reaction, the total mass and total atomic number remains the same.
For the given fission reaction:
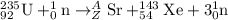
To calculate A:
Total mass on reactant side = total mass on product side
235 + 1 = A + 143 + 3
A = 90
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
92 + 0 = Z + 54 + 0
Z = 38
Hence, the isotopic symbol of strontium is:-
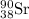
The element given is:-
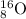
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
Also, atomic number of O = 8
Thus, the number of protons = 8
Mass number is the number of the entities present in the nucleus which is the equal to the sum of the number of protons and electrons.
Mass number = Number of protons + Number of neutrons
16 = 8 + Number of neutrons
Number of neutrons = 8
Mass of neutron = 1.008665 amu
Mass of proton = 1.007825 amu
Calculated mass = Number of protons*Mass of proton + Number of neutrons*Mass of neutron
Thus,
Calculated mass = (8*1.007825 + 8*1.008665) amu = 16.13192 amu
Given, mass of the atom = 15.994914 amu
Mass defect = Δm = |16.13192 - 15.994914| amu = 0.137006 amu
Mass defect of the oxygen nucleus is:- 0.1370 amu