Answer:
The efficiency of this engine is 37.63 %.
Step-by-step explanation:
Given;
useful output work done by the combustion engine, = 356 kJ
input work, = 946 kJ
The efficiency of the combustion engine is calculated as;
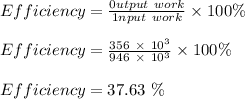
Therefore, the efficiency of this engine is 37.63 %.