Answer:
W= 4.89 KJ
Step-by-step explanation:
Lets take
temperature of hot water T₁ = 100⁰C
T₁ = 373 K
Temperature of cold ice T₂= 0⁰C
T₂ = 273 K
The latent heat of ice LH= 334 KJ
The heat rejected by the engine Q= m .LH
Q₂= 0.04 x 334
Q₂= 13.36 KJ
Heat gain by engine = Q₁
For Carnot engine
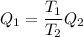
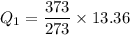
Q₁ = 18.25 KJ
The work W= Q₁ - Q₂
W= 18.25 - 13.36 KJ
W= 4.89 KJ