Step-by-step explanation:
Osmotic pressure is defined as the minimum pressure that has to be applied to a solution in order to prevent the inward flow of the pure solvent across a semipermeable membrane.
The formula used to calculate the osmotic pressure of the solution follows:
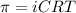
where,
C = molarity of the solution
T = absolute temperature of the solution
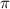 = osmotic pressure exerted by a solution
= osmotic pressure exerted by a solution
i = Van't Hoff factor or the ions present in the solution