Answer:
W= 8120 KJ
Step-by-step explanation:
Given that
Process is isothermal ,it means that temperature of the gas will remain constant.
T₁=T₂ = 400 K
The change in the entropy given ΔS = 20.3 KJ/K
Lets take heat transfer is Q ,then entropy change can be written as
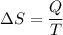
Now by putting the values
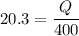
Q= 20.3 x 400 KJ
Q= 8120 KJ
The heat transfer ,Q= 8120 KJ
From first law of thermodynamics
Q = ΔU + W
ΔU =Change in the internal energy ,W=Work
Q=Heat transfer
For ideal gas ΔU = m Cv ΔT]
At constant temperature process ,ΔT= 0
That is why ΔU = 0
Q = ΔU + W
Q = 0+ W
Q=W= 8120 KJ
Work ,W= 8120 KJ