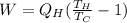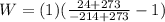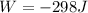To solve this problem it is necessary to apply the concepts related to the coefficient of performance (COP) of a heat pump, cooling or an air conditioning system. Said performance coefficient basically expresses the radius of useful heating or cooling provided to work required. At this case we use the expression for a Refrigerator.
Mathematically it can be expressed as
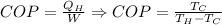
Where
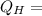 Heat of hot reservoir
Heat of hot reservoir
W = Work
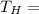 Temperature of hot reservoir
Temperature of hot reservoir
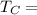 Temperature of cold reservoir
Temperature of cold reservoir
Matching the two equations we have to
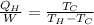
Re-arrange to find the Work
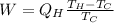
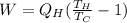
Our values depend on each point but for all the heat that is equivalent to 1 remains the same, therefore replacing for each one,
PART A)
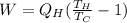
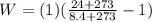
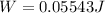
PART B)
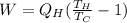
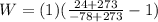
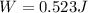
PART C)
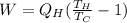
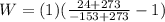
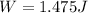
PART D)