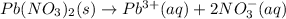Answer :
(b) The balanced overall ionic equation will be,
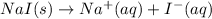
(c) The balanced overall ionic equation will be,
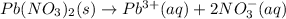
Explanation :
Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and present in an aqueous are represented in the form of ions.
Net ionic equation : In the net ionic equations, we are not include the spectator ions in the equations.
Spectator ions : The ions present on reactant and product side which do not participate in a reactions. The same ions present on both the sides.
Part B :
When sodium iodide dissolving in water then it dissociates to give sodium ion and iodide ion.
The balanced overall ionic equation will be,
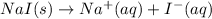
Part C :
When lead(II) nitrate dissolving in water then it dissociates to give lead ion and nitrate ion.
The balanced overall ionic equation will be,