Answer:
2,296.7 grams of water can be cooled from 35°C to 20 °C by the evaporation of 60 g of water.
Step-by-step explanation:
The heat of evaporation of water =
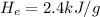
Mass of water evaporated , m= 60 g
Amount of heat required to evaporate 60 grams of water :Q
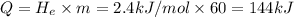
Amount of heat lost to condense water 35 to 20 °C = -Q = -144 kJ = 144,000 J
Mass of water = m
Specific hat of water = c = 4.18 J/g K
Initial temperature =
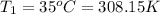
Final temperature =
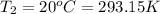
Change in temperature =
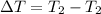
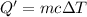
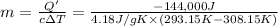
m = 2,296.7 g
2,296.7 grams of water can be cooled from 35°C to 20 °C by the evaporation of 60 g of water.