The concept related to this exercise to solve this problem is the ideal Gas law which establishes
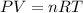
P= Pressure
V= Volume
n = Number of moles
R= Gas ideal Constant
T= Temperature
Our values are given as,
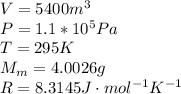
From the ideal gas equation then we rearrange the equation to obtain the number of moles, then
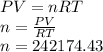
By definition the molecular mass (n) is expressed in terms of the mass and molecular weight therefore
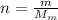
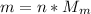
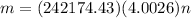
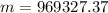
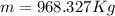
Therefore the mass of the helium in the blimp is 968.327Kg